About
We Are Cellmyx
Cellmyx is committed to advancing the art of aesthetic and regenerative injectables.
cosmetic surgery products| Cannulas | Liposuction | fat grafting |autologous
We design, patent, and manufacture proprietary medical products for the harvesting, processing, and deployment of FDA 361 & FDA 510 compliant autologous soft tissue.
We continue to explore novel concepts to enhance the development of our proprietary product portfolio. Visit the Blog for the latest news.
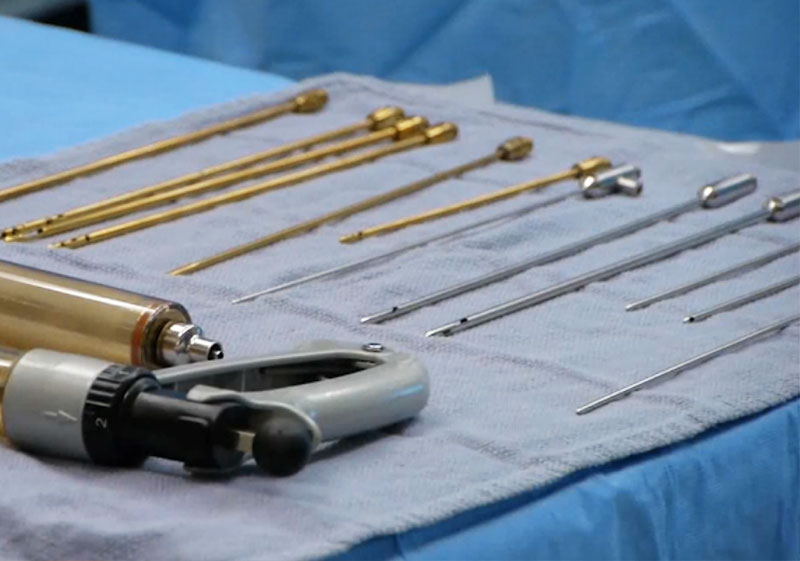
Technologies
We started with canisters, cannulas and tubing used during liposuction cases. We have now grown to developing products like our SuperG® Cannula Series, AcquiCell™, Lipo-Loop®, and now intelliFat™ with 3 more products in the development pipeline.
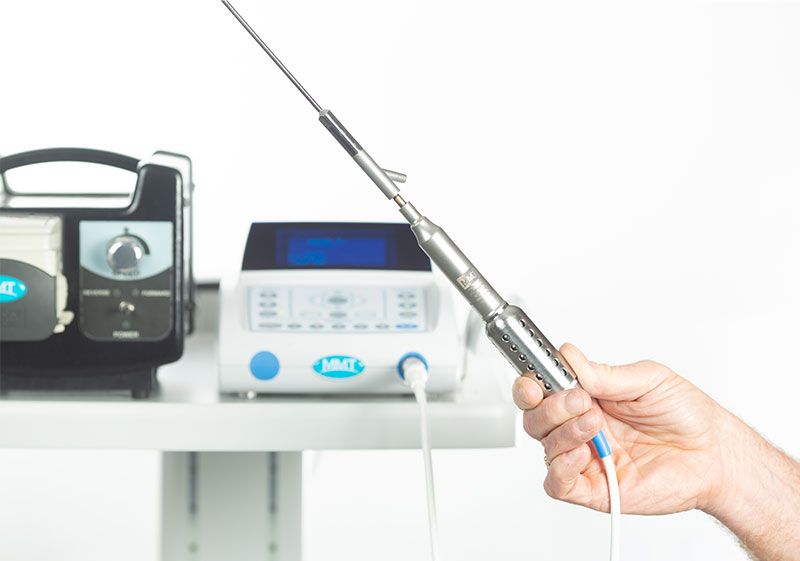
Intellectual Leadership
We specialize in the fields of cosmetic surgery and regenerative medicine. From concept, to validation, to manufacturing, we are meticulous in how, and passionate in why we develop the products we do.

Client Support
We pride ourselves in the first-class support we provide to our customers. Visit our Intelligent Injectables Advisory Board or Contact Us directly if you have clinical or scientific questions about our products, or if you require support.
History
Founded in 1997
by Current Founder & CEO Greg Miles
Millennium Medical Technologies, Inc. (“MMT”), a medical device and development company, was founded in California.
2000: Consulting for ArthoCleanse®
Granted U.S. Patent US No. 6066146 A
MMT provided consulting for Advanced Diagnostics & Interventions, Inc. for a product called ArthoCleanse® with granted U.S. Patent US No. 6066146 A.
2003: Began Developing Product Lines
To be used in cosmetic surgery applications
MMT began developing products in this market segment in 2003 when the company filed its first establishment registration with the FDA.
2008: FDA Premarket Notification 510(k)
Liposuction Cannulas (K081039O)
Filed with the U.S. Food and Drug Administration (“FDA”) a Premarket Notification 510(k) for the manufacture of liposuction cannulas (K081039O) in September 2008.
First Initiative with Arthroscopic Products
Arthroscopic Knee Cannula System Development
Assisted arthroscopic products manufacturer Linvatec to develop a new arthroscopic knee cannula system. Linvatec was subsequently acquired by medical products manufacturer CONMED Corporation, and the cannula system we helped develop has grown to an annual sales level of $20 million.
2012: Cell Solutions™
With Power-Assisted Lipo as the Foundation
A service-based system for point of care autologous cell therapy which follows Good Tissue Practice Guidance. We created a custom regenerative aesthetic and medicine package that meets the need of the physicians specialty be that plastic surgery, orthopedics or pain management.
2015: Developed AcquiCell™
A New Approach to Soft Tissue Harvesting and Transfer
Developed in July 2015, the AcquiCell™ device is ideal for physicians involved with tissue grafting and regenerative medicine using adipose and stromal cells.
2017: FDA Clearance Granted
Fat Collection, Transfer and Disposable Systems (K170449)
510(k) clearance granted on the first fully reusable fat collection, transfer and disposable systems (K170449) in March 2017. In conjunction with tubing for canister systems, MMT developed a closed-loop system called Lipo-Loop®.
2018: Cellmyx
MMT becomes Cellmyx
Millennium Medical Technologies, Inc. changes its name to Cellmyx, Inc.lled Lipo-Loop®.
2018: intelliFat™
intelliFat™ Kit Launch
Cellmyx introduces the intelliFat™ Kit – an innovative fat grafting kit that enables physicians to quickly and efficiently transfer the patient’s own adipose tissue under local anesthesia. All in a single use, disposable kit. The intelliFat™ makes low volume fat transfers fast, simple, and cost effective.
2019: intelliFat™
intelliFat™ SVT Kit Launch
intelliFat™ SVT is a new iteration of our popular intelliFat™ kit that enables physicians to quickly and efficiently transfer the patient’s own micro-adipose tissue under local anesthesia for regenerative procedures.
2020: SuperG® Micro Cannula
Directly harvest viable microfat without the need for using harmful mechanical processes.
Optimized for harvesting 500µm clusters that are perfect for fat transfer to the whole face.
2020: New Online Store
Cellmyx launches new online store
You will be able to securely purchase a wide range of surgical devices and disposables used in liposculpture, fat collection, and fat transfer.
